Botulinum Toxin A Emerges As Promising Treatment For Female Pattern Hair Loss: Study
- byDoctor News Daily Team
- 06 July, 2025
- 0 Comments
- 0 Mins
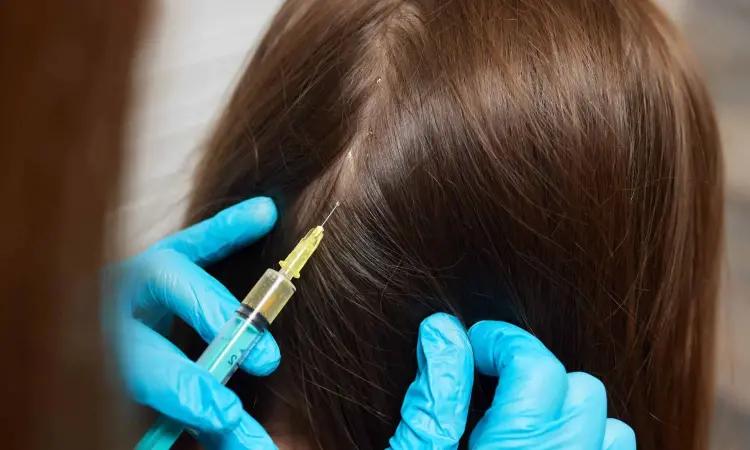
China: In a breakthrough discovery in dermatology, a recent study has illuminated the potential of botulinum toxin A as a novel therapeutic option for female pattern hair loss (FPHL). The findings, published in Skin Research and Technology, offer new hope for individuals grappling with this distressing condition and signify a paradigm shift in hair loss management.
The study suggested the effectiveness of Botulinum Toxin Type A (BTA) for female pattern hair loss is limited to three months. However, it can be considered for tentative use after effective communication with patients. Further observation and study are needed for BTA's long-term safety and efficacy in treating FPHL.
Female pattern hair loss, characterized by progressive hair thinning primarily over the crown and frontal scalp regions, affects a significant proportion of women worldwide, exerting profound psychological and emotional impact. Despite its prevalence, treatment options have traditionally been limited, with topical minoxidil and oral medications like spironolactone comprising the mainstay of therapy.
However, the emergence of botulinum toxin A, commonly known for its cosmetic applications in wrinkle reduction, heralds a promising alternative for FPHL sufferers. Xiuzu Song, The Department of Dermatology, Hangzhou Third People's Hospital, Hangzhou City, China, and colleagues aimed to assess the safety and efficacy of subcutaneous injections of Botulinum Toxin Type A in FPHL treatment.
The study included outpatients with FPHL who exhibited an allergic reaction to minoxidil solution. FPHL diagnosis was established through trichoscopy and clinical examination. Inclusion criteria involved patients with no prior treatment within the last year and without comorbidities.
BTA, specifically 100 units, was mixed with 2 mL of 0.9% normal saline. Twenty injection target sites, spaced 2–3 cm apart, were symmetrically marked on the scalp's hairless area. At each target site, a dosage of five units was injected intradermally. Scalp's representative photographs and dermoscopic images were captured before and after three months of treatment.
The study included ten women with FPHL, aged between 26 and 40 years. The average age was 30.3 ± 4.64 years, and all patients had a positive family history of Androgenetic Alopecia. The average disease duration was 3.70 ± 1.42 years.
The study led to the following findings:
According to patients' self-assessment, after one month of treatment, 10 FPHL patients reported experiencing moderate to marked improvement in symptoms related to scalp oil secretion.
Three months later, dermatological assessments showed that three had mild improvement, six had no change, and one had a worsening condition.
No adverse effects were observed.
The study showed that the effectiveness of Botulinum Toxin Type A for treating Female Pattern Hair Loss treatment is limited to 3 months. However, it can be considered for tentative use after effective communication with patients.
The study limitations were the small sample size of this experiment, FPHL patients of different severity have not been grouped and stratified compared, and for a short follow-up period.
"The long-term safety and efficacy of BTA in treating FPHL require further observation and study," the researchers concluded.
Reference:
Hu, L., Dai, Y., Zhang, H., Wu, Y., Wang, T., & Song, X. (2024). Efficacy and safety of botulinum toxin A in the treatment of female pattern hair loss. Skin Research and Technology, 30(4), e13696. https://doi.org/10.1111/srt.13696
Disclaimer: This website is designed for healthcare professionals and serves solely for informational purposes.
The content provided should not be interpreted as medical advice, diagnosis, treatment recommendations, prescriptions, or endorsements of specific medical practices. It is not a replacement for professional medical consultation or the expertise of a licensed healthcare provider.
Given the ever-evolving nature of medical science, we strive to keep our information accurate and up to date. However, we do not guarantee the completeness or accuracy of the content.
If you come across any inconsistencies, please reach out to us at
admin@doctornewsdaily.com.
We do not support or endorse medical opinions, treatments, or recommendations that contradict the advice of qualified healthcare professionals.
By using this website, you agree to our
Terms of Use,
Privacy Policy, and
Advertisement Policy.
For further details, please review our
Full Disclaimer.
Recent News
Pfizer files lawsuit against Metsera, its Director...
- 02 November, 2025
Health Ministry achieves 3 Guinness World Records...
- 02 November, 2025
Roche gets CE mark for Elecsys Dengue Ag test to d...
- 02 November, 2025
Daily Newsletter
Get all the top stories from Blogs to keep track.


0 Comments
Post a comment
No comments yet. Be the first to comment!