Tralokinumab Monotherapy Effective In Moderate-To-Severe Atopic Dermatitis: Study
- byDoctor News Daily Team
- 17 July, 2025
- 0 Comments
- 0 Mins
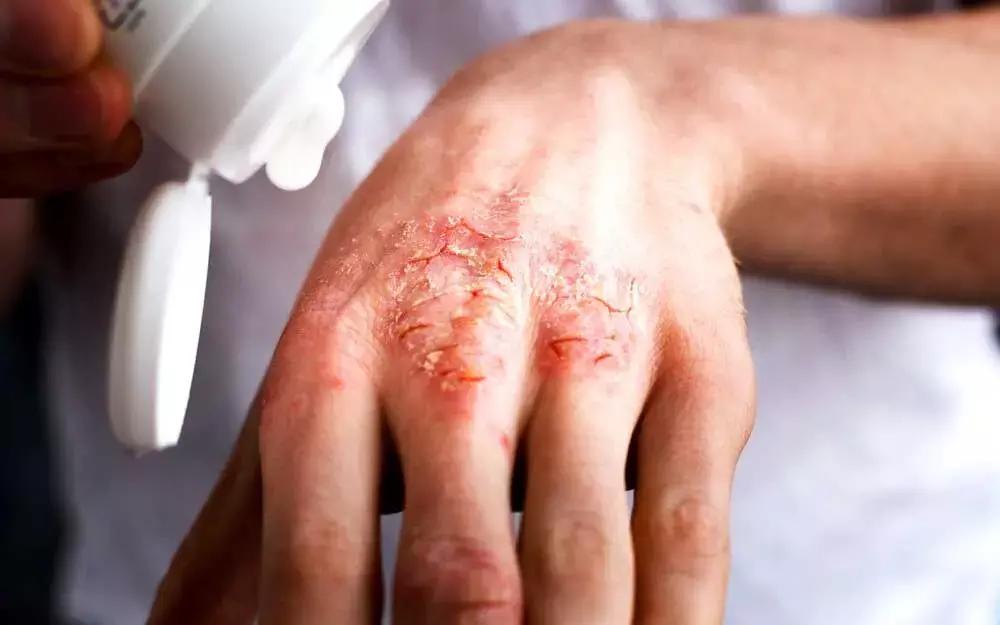
Recent evidence shows that Tralokinumab monotherapy was superior to placebo at 16 weeks of treatment and was well tolerated up to 52 weeks of treatment for patients with moderate‐to‐severe atopic dermatitis.
This study is published in the British Journal of Dermatology.
Tralokinumab, a fully human monoclonal antibody, specifically neutralizes interleukin‐13, a key cytokine driving peripheral inflammation in atopic dermatitis (AD). In phase II studies, tralokinumab combined with topical corticosteroids provided early and sustained improvements in AD signs and symptoms.
Hence, A. Wollenberg and colleagues from the Department of Dermatology and Allergy, Ludwig Maximilian University of Munich, Munich, Germany conducted the study to evaluate the efficacy and safety of tralokinumab monotherapy in adults with moderate‐to‐severe AD who had an inadequate response to topical treatments.
In two 52‐week, randomized, double‐blind, placebo‐controlled, phase III trials, ECZTRA 1 and ECZTRA 2, adults with moderate‐to‐severe AD was randomized (3: 1) to subcutaneous tralokinumab 300 mg every 2 weeks (Q2W) or placebo. Primary endpoints were Investigator's Global Assessment (IGA) score of 0 or 1 at week 16 and ≥ 75% improvement in Eczema Area and Severity Index (EASI 75) at week 16. Patients achieving an IGA score of 0 or 1 and/or EASI 75 with tralokinumab at week 16 were rerandomized to tralokinumab Q2W or every 4 weeks or placebo, for 36 weeks.
The results showed-
a. At week 16, more patients who received tralokinumab vs. placebo achieved an IGA score of 0 or 1: 15·8% vs. 7·1% in ECZTRA 1 [difference 8·6%, 95% confidence interval (CI) 4·1–13·1; P = 0·002] and 22·2% vs. 10·9% in ECZTRA 2 (11·1%, 95% CI 5·8–16·4; P < 0·001) and EASI 75: 25·0% vs. 12·7% (12·1%, 95% CI 6·5–17·7; P < 0·001) and 33·2% vs. 11·4% (21·6%, 95% CI 15·8–27·3; P < 0·001).
b. Early improvements in pruritus, sleep interference, Dermatology Life Quality Index, SCORing Atopic Dermatitis and Patient‐Oriented Eczema Measure was observed from the first postbaseline measurements.
c. The majority of week 16 tralokinumab responders maintained response at week 52 with continued tralokinumab treatment without any rescue medication (including topical corticosteroids).
d. Adverse events were reported in 76·4% and 61·5% of patients receiving tralokinumab in ECZTRA 1 and ECZTRA 2, respectively, and in 77·0% and 66·0% of patients receiving placebo in ECZTRA 1 and ECZTRA 2, respectively, in the 16‐week initial period.
Hence, Tralokinumab monotherapy was superior to placebo at 16 weeks of treatment and was well tolerated up to 52 weeks of treatment, the authors concluded.
Disclaimer: This website is designed for healthcare professionals and serves solely for informational purposes.
The content provided should not be interpreted as medical advice, diagnosis, treatment recommendations, prescriptions, or endorsements of specific medical practices. It is not a replacement for professional medical consultation or the expertise of a licensed healthcare provider.
Given the ever-evolving nature of medical science, we strive to keep our information accurate and up to date. However, we do not guarantee the completeness or accuracy of the content.
If you come across any inconsistencies, please reach out to us at
admin@doctornewsdaily.com.
We do not support or endorse medical opinions, treatments, or recommendations that contradict the advice of qualified healthcare professionals.
By using this website, you agree to our
Terms of Use,
Privacy Policy, and
Advertisement Policy.
For further details, please review our
Full Disclaimer.
Tags:
Recent News
Eli Lilly plans to build new USD 3 billion facilit...
- 04 November, 2025
Rajkot Maternity Hospital CCTV Leak: How a simple...
- 04 November, 2025
Gland Pharma profit rises 12 percent to Rs 184 cro...
- 04 November, 2025
AIIMS Delhi doctors told to use Hindi in prescript...
- 04 November, 2025
Daily Newsletter
Get all the top stories from Blogs to keep track.


0 Comments
Post a comment
No comments yet. Be the first to comment!