TCT 2025: OCVC-BIF Trial Compares Drug-Coated Balloon Versus Conventional Balloon for Treatment of Side Branches in Coronary Bifurcation Lesions
- byDoctor News Daily Team
- 29 October, 2025
- 0 Comments
- 0 Mins
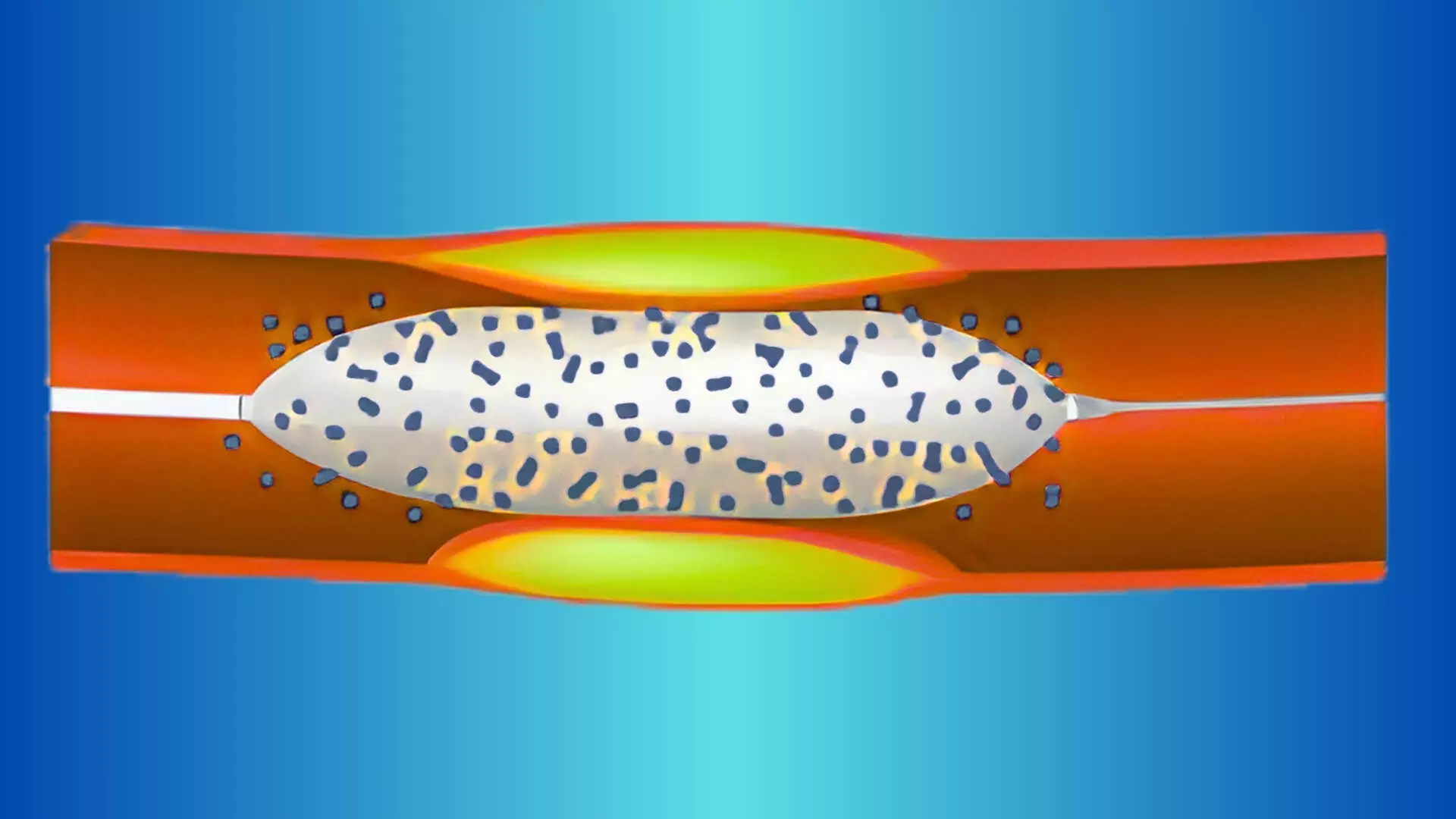
This news is covered by the Bureau present at the TCT Conference 2025, being held in San Francisco, USA. Additional drug-coated balloon (DCB) inflation in the side branch following main-vessel drug-eluting stent (DES) implantation with kissing balloon inflation (KBI) significantly reduced side branch restenosis at one year compared to without additional DCB inflation, according to results from the OCVC-BIF randomized trial presented by Dr. Takayuki Ishihara on behalf of the Osaka Cardiovascular Conference (OCVC) investigators at TCT 2025. The multicenter, open-label OCVC-BIF study was conducted across eight hospitals within the Osaka CardioVascular Conference network to evaluate the efficacy and safety of DCB use in the side branch of coronary bifurcation lesions—a subset that accounts for 15–20% of all coronary lesions and remains technically challenging with higher rates of adverse events. While DCBs have demonstrated favorable results in small vessel disease, their role in bifurcation lesions, particularly for side branches, has remained uncertain. A total of 299 patients undergoing percutaneous coronary intervention (PCI) for bifurcation lesions suitable for single-stent strategy (DES in the main vessel with KBI) were randomized to DCB inflation for the side branch (n=149) or no additional DCB (n=145). Eligible lesions had side branch diameters between 2.0 and 3.0 mm, and follow-up coronary angiography was performed at nine months. The primary endpoint was side branch restenosis, defined as ≥50% stenosis on three-dimensional bifurcation quantitative coronary angiography (QCA) at nine months or symptom-driven angiography within one year. At one year, the DCB strategy resulted in significantly lower side branch restenosis compared with the non-DCB group (8.1% vs. 18.3%; odds ratio 0.36, 95% CI 0.16–0.79; p=0.012). Mean side branch diameter stenosis at follow-up was also lower with DCB treatment (32.5% vs. 36.2%; p=0.03), and minimal lumen diameter was greater (1.45 mm vs. 1.32 mm; p=0.02). Late lumen loss in the main vessel was less in the DCB group (0.21 mm vs. 0.36 mm; p=0.037). Secondary endpoints, including clinically driven target lesion revascularization (CD-TLR), procedural success, and major adverse cardiac events (MACE), were similar between groups. CD-TLR occurred in 0.7% (n=1) of DCB-treated patients versus 3.4% (n=5) in the control group (p=0.20), while one-year MACE rates were 10.1% and 12.4%, respectively (p=0.65). There were no cases of cardiac death or stent thrombosis in either arm. Investigators concluded that adjunctive DCB inflation for the side branch after DES implantation with KBI can effectively reduce angiographic restenosis without increasing procedural risk. However, they noted that the study was limited by its moderate sample size, low representation of female patients, and use of a single paclitaxel-coated balloon platform. Longer-term follow-up and evaluation with newer-generation sirolimus-coated balloons are warranted. The OCVC-BIF trial provides new randomized evidence supporting a DCB-based approach for optimizing outcomes in bifurcation PCI, particularly in preserving side branch patency while maintaining procedural simplicity and safety. Reference:Takayuki Ishihara et al., Comparison of Drug-Coated Versus Conventional Balloons for Treatment of Side Branches of Bifurcation Lesions- The OCVC-BIF Study, TCT Conference 2025, San Francisco. https://www.tctconference.com/ About the Study Presenter:Takayuki Ishihara is an interventional cardiologist at Kansai Rosai Hospital - Amagasaki, Japan. His areas of interest include complex coronary interventions, atrial fibrillation, ablation, impact of kidney disease on cardiac procedures, and long-term prognosis after cardiac treatments.
Disclaimer: This website is designed for healthcare professionals and serves solely for informational purposes.
The content provided should not be interpreted as medical advice, diagnosis, treatment recommendations, prescriptions, or endorsements of specific medical practices. It is not a replacement for professional medical consultation or the expertise of a licensed healthcare provider.
Given the ever-evolving nature of medical science, we strive to keep our information accurate and up to date. However, we do not guarantee the completeness or accuracy of the content.
If you come across any inconsistencies, please reach out to us at
admin@doctornewsdaily.com.
We do not support or endorse medical opinions, treatments, or recommendations that contradict the advice of qualified healthcare professionals.
By using this website, you agree to our
Terms of Use,
Privacy Policy, and
Advertisement Policy.
For further details, please review our
Full Disclaimer.
Recent News
AIIMS INI SS January 2026: 4 seats added in 2 spec...
- 01 November, 2025
Treatment in Myocardial Infarction and Non-Obstruc...
- 01 November, 2025
PG medical admissions 2025 commence in Bihar, chec...
- 01 November, 2025
Assam to begin NEET PG 2025 counselling from Novem...
- 01 November, 2025
Daily Newsletter
Get all the top stories from Blogs to keep track.


0 Comments
Post a comment
No comments yet. Be the first to comment!