Loteprednol Etabonate Formulated With MPP Technology Effective In Dry Eye Disease
- byDoctor News Daily Team
- 28 July, 2025
- 0 Comments
- 0 Mins
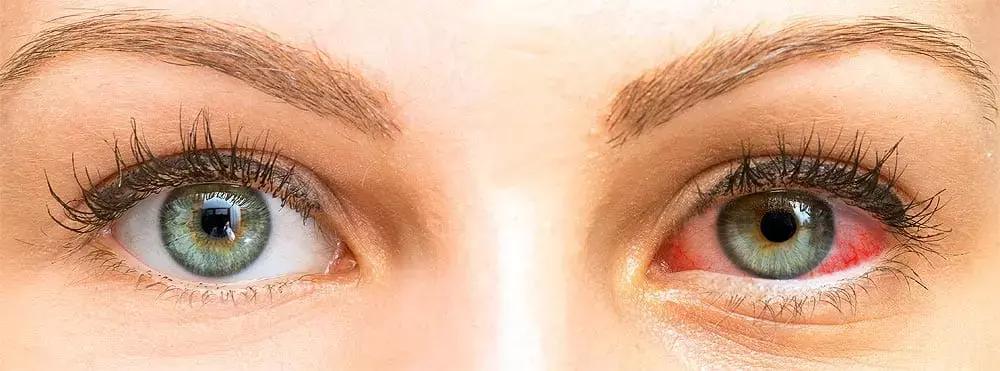
USA: A review, published in the Dove press journal Clinical Ophthalmology, has described the use of a commercially available formulation of loteprednol etabonate ophthalmic suspension (0.25%) for the treatment of acute symptoms of dry eye disease (DED). It found that the unique formulation is well-suited for dry eye flare-ups.
Dry eye disease is a prevelant disease of ocular surface. Patients with DED can get episodic flares, like any chronic disease. For the chronic treatment of DED, there are many existing and upcoming treatments, yet treatments for DED flares are limited. Loteprednol etabonate 0.25% is approved by FDA for the short-term treatment of the signs and symptoms of DED.
Formulation of this medications is with the customized mucus-penetrating particle (MPP) technology that has a significant ability to penetrate the ocular surface for effective deliver of the active steroid to the ocular surface tissues as compared with conventional steroid preparations. The MPP technology involves engineering nanoparticles designed to effectively penetrate mucus and prevent entrapment of drug particles by mucins. Also, there is an increasing use of loteprednol etabonate 0.25% for the treatment of DED before and/or after cataract or refractive surgery or as induction therapy before starting chronic immunomodulatory medication for DED.
There has been a focus on understanding the disease pathophysiology and identifying effective treatment modalities given the significant prevalence and morbidity of DED.
Nandini Venkateswaran, Massachusetts Eye and Ear Infirmary, Harvard Medical School, Boston, MA, USA, and colleagues conclude, "KP-121 0.25% with its custom engineered MPP technology allows for the effective and efficient delivery of loteprednol etabonate onto the ocular surface for the short-term treatment of signs and symptoms of DED with minimal side effects."
"Clinical trials have demonstrated the utility of this medication in DED flares and clinicians should incorporate this medication into their DED treatment armamentarium," they wrote.
Reference:
Venkateswaran N, Bian Y, Gupta PK. Practical Guidance for the Use of Loteprednol Etabonate Ophthalmic Suspension 0.25% in the Management of Dry Eye Disease. Clin Ophthalmol. 2022;16:349-355
DOI: https://doi.org/10.2147/OPTH.S323301
Disclaimer: This website is designed for healthcare professionals and serves solely for informational purposes.
The content provided should not be interpreted as medical advice, diagnosis, treatment recommendations, prescriptions, or endorsements of specific medical practices. It is not a replacement for professional medical consultation or the expertise of a licensed healthcare provider.
Given the ever-evolving nature of medical science, we strive to keep our information accurate and up to date. However, we do not guarantee the completeness or accuracy of the content.
If you come across any inconsistencies, please reach out to us at
admin@doctornewsdaily.com.
We do not support or endorse medical opinions, treatments, or recommendations that contradict the advice of qualified healthcare professionals.
By using this website, you agree to our
Terms of Use,
Privacy Policy, and
Advertisement Policy.
For further details, please review our
Full Disclaimer.
Recent News
What Your Neck Size Says About Your Heart Health?...
- 04 November, 2025
EVOQUE TTVR Delivers Promising Real-World Results:...
- 04 November, 2025
Influenza Vaccination Reduces Mortality and Readmi...
- 04 November, 2025
Can Technology Make Kids Healthier? New Research S...
- 04 November, 2025
Daily Newsletter
Get all the top stories from Blogs to keep track.


0 Comments
Post a comment
No comments yet. Be the first to comment!